We are excited to share with you a new development in bringing a therapy for x-linked hypohidrotic ectodermal dysplasia (XLHED) to clinical trial. EspeRare announced today that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy Designation to the protein replacement therapy called ER-004.

FDA gives this designation to expedite the development and review of drugs for serious or life-threatening conditions. Essentially, it gives this XLHED research project fast-track privileges such as FDA guidance on an optimal drug development plan and FDA’s commitment from senior managers.
Having the designation will speed up the process to bring this potential therapy to our families affected by XLHED. We couldn’t be happier about that!
“This is extraordinary news for our ectodermal dysplasias community in the United States and around the world,” Mary Fete, NFED Executive Director. “The NFED led the effort for three decades to develop a treatment for XLHED. Having the FDA Breakthrough Therapy Designation means scientists can aspire starting a clinical trial to confirm the findings for ER-004 and ultimately, if successful, make this treatment an option for families. It brings us closer to an amazing time when children born with XLHED could have working sweat glands! We are honored to work with EspeRare to make this happen.”

Caroline Kant, CEO of EspRare, commented:
Read EspeRare Announcement and Watch Video Read EspeRare’s announcement in German Read EspeRare’s Announcement in French“Obtaining the Breakthrough Therapy designation for ER-004 has been made possible by the efforts of the NFED and the XLHED patient community at large over the last 30 years. EspeRare feels incredibly fortunate to be working hand in hand with this community; it is such exciting time to progress this innovative therapy that has the potential to fundamentally change the lives of XLHED patients.”
The Road to Designation

The FDA granted the designation based on the findings from when Prof. Holm Schneider successfully treated three males affected by XLHED with ER-004 while in-utero. ER-004 is a bio-engineered protein that replaces the nonfunctioning protein missing in the EDA gene that causes XLHED.
ER-004 protein was injected into the amniotic fluid in the third trimester of the pregnancy. Each of the infants experienced life-changing results including working sweat glands, an increased number of tooth buds and no respiratory issues. Prof. Schneider published these findings in the New England Journal of Medicine.
The Next Study
EspeRare’s future study seeks to confirm Prof. Schneider’s findings in a larger number of individuals. If successful, the treatment would be brought to market! They anticipate this study launching in the second half of 2021 in Europe and then the United States.
According to EspeRare, “In the U.S., in addition to this FDA Breakthrough Therapy the program benefits from Fast Track and Orphan Drug Designation. In Europe, the program receives support from the EMA’s PRIME (Priority Medicines) and also the Orphan Drug Designation. With these multiple regulatory incentives, the program can aim at a streamlined worldwide development.”
Shape Our Futures With Research
Ectodermal dysplasia can cause a lifetime of challenges. By supporting research, you expand early diagnostics, treatments, pathways toward cures… and hope!
Donate to ResearchFirst of Its Kind
We know that the study will launch later than we had originally thought. But, that is often the case with research and drug development. And, it certainly has held true for XLHED research.
Since the NFED initiated the first research efforts in the late 1980s, the journey from gene identification and ER-004 development to animal testing and the Newborn XLHED Clinical Trial, there have been many stops and starts.
But, we keep working and pushing forward. And our efforts have led to this upcoming important study. The NFED is working with EspeRare to provide support in any way we can to advance the research.
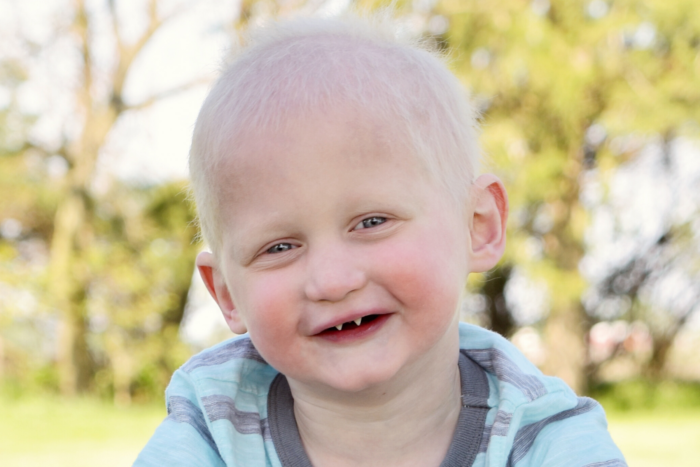
Today’s announcement is another important milestone to celebrate on our way to a therapy. If ER-004 works, it could be the first therapy to treat a genetic condition before birth!
Learn More about XLHED Research Studies
This is amazing… I’m a carrier and my son has HED. As we get older we sweat. I’ve always wondered if there was a way to get the sweat glands working earlier.
Hi, Sandra. We learned from the research that the treatment has to happen before the sweat glands every form which is in utero. It worked for three boys and hoping it works in the upcoming research EspeRare hopes to launch! ~ Jodi, Director, Marketing and Communications, NFED
There are no words to adequately express my joy over this news. Hurray! Hurray!!
We share your joy, Mary Kaye, and thank you for starting this effort all those years ago. It was your vision!
This is why I give every month! The dedication of this organization is amazing in assisting with getting legislation and organizations involved in finding help for this disorder. I have lived with it all of my life and didn’t understand it for a long time (and I am still learning). This is exciting news for future generations!!
Thank you for being such a dedicated supporter, Kimberly! Together, we are able to accomplish much more than we ever could alone. Our families have helped us advance this research by volunteering and donating every step of the way! We couldn’t do it without all of you! And, it is incredibly exciting news!
From Nigeria. Infact words are not enough to express for the effort you guys as put on. Congratulation for the breakthrough in your effort.
My little boy is 2 years old can anything be done too help him sweat . he has only one pointy tooth ,and very sparce hair ,we did not know about the injection before he was born . and if there is something can be done too help him now .
My little boy is 2 years old can anything be done too help him sweat . he has only one pointy tooth ,and very sparce hair ,we did not know about the injection before he was born . and if there is something can be done too help him now .
Please contact our office at info@nfed.org and someone can provide you with resources. Thank you!