
By Beth Orchard
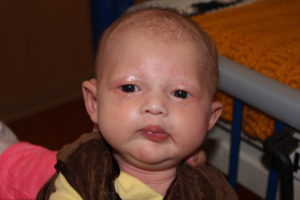
My husband and I enrolled our two-month-old son, Liam, in the XLHED Newborn Clinical Trial less than two weeks after he was born.
Even before pregnancy I knew about the study and, if we had an affected son, would enroll him. There are many reasons but the greatest one was this: to give him a chance at a better life.
Although I’m mildly affected (by x-linked hypohidrotic ectodermal dysplasia or XLHED), we didn’t know whether our son would be. As it turns out he may also have more mild symptoms but will still not get many of his baby teeth and will not sweat like other kids.
Our decision to participate rested in a strong faith and hope we have that this research will not only benefit him but future children born with XLHED. Knowing our son, at such a young age, was contributing to a cause greater than himself was another big reason we chose to participate.
The other reason we enrolled Liam was because of our NFED family. The NFED’s Treatment Assistance Program helped me cover costs of getting my implants, crowns and necessary dental work done. Their resources have helped me and my family learn about mine and other ectodermal dysplasias as well as Edimer’s research opportunity, ways to engage with others similarly affected and provide love and support (as any family does).

In fact, three of my “family members” from NFED came to visit us in St. Louis during the clinical trial to lend support and a shoulder to lean on. Without the NFED family, I wouldn’t be as strong and confident as I am that I can tackle XLHED head on and now support my son as he does the same.
“Strive not to be a success, but rather to be of value.” –Albert Einstein
Beth’s blog is a part of our Helping Hand series featuring incredible NFED volunteers. learn more on our website about volunteering.

[…] Why I Participated in the Edimer Research Trials […]
[…] Why I Volunteered to Participate in the Edimer Research Trials […]
[…] Why I Volunteered to Participate in the Edimer Research Trials […]
[…] Why I Participated in the Edimer Research Trials […]
How can i diagnose that case before birth as i have two similar cases in my family and im now pregnant in 33 weeks
Edimer Pharmaceuticals is no longer providing genetic testing since their clinical trial is not accepting patients at this time. You can have genetic testing performed. You need to see your physician or a geneticist who can order the appropriate tests. GeneDx.org is one genetics lab who can test. However, a doctor must order the specific test needed based on your family history and clinical diagnosis.