The EDELIFE clinical trial is investigating a potential treatment for boys affected by x-linked hypohidrotic ectodermal dysplasia (XLHED). Some of you have asked why the clinical trial is focused only on boys and not girls with XLHED. Let us explain.
The answer is directly related to how XLHED is inherited, or passed down from parents to children, and the best practices for studying potential drugs. This includes having consistent baselines to measure symptom reduction and ensuring the benefit of the treatment outweighs any potential risk to the participant. Once the treatment is approved for use in boys, it will be considered for use in girls as well.
Genetics
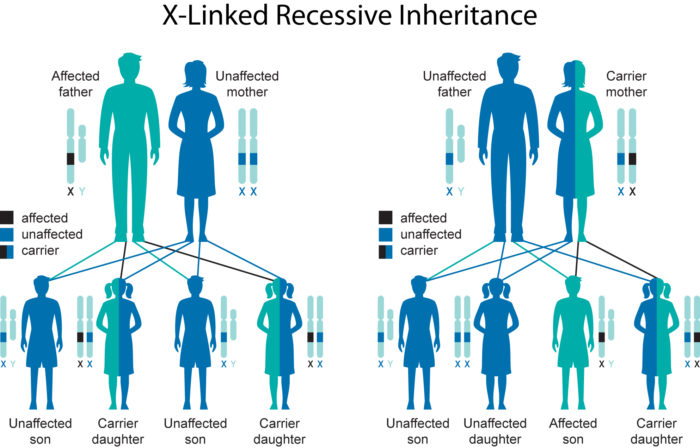
The gene responsible for XLHED is the ectodysplasin (EDA) gene, which is located on the X chromosome. The EDA gene is responsible for the production of the protein ectodysplasin A1 (EDA1), which signals the development of ectodermal structures (such as hair, teeth, skin, sweat glands, etc.). In XLHED, the EDA gene is faulty and does not produce working EDA1, leading to the symptoms of XLHED.

Boys only have one X chromosome, and if they inherit the faulty one from their mothers, they will not be able to make any EDA1 and will be fully affected by XLHED, showing the full range of symptoms.

Girls on the other hand, have two X chromosomes, and if they inherit a faulty one, the issues they would experience can be partially compensated by the other fully functioning X chromosome.
This is why XLHED symptoms in girls vary and may be mild to non-existent and is why boys with XLHED are always affected and consistently more so than girls.
Benefit/Risk Concept in Drug Approval
Drugs can provide a significant relief, but they also carry risks. For a new drug to be approved by the health authorities, such as the Food and Drug Administration (FDA), the criteria is always what is known as the “risk/benefit balance.” In short, this means that a drug must demonstrate that it provides a substantial benefit, such that it outweighs the risks of giving it.
Boys with XLHED are consistently severely affected. They represent a homogenous population, or group where the individuals have the same genetic makeup and symptoms. Therefore, the consistent expression of symptoms in boys means we can demonstrate if a response is directly linked to the drug.
In girls, there is variability of the observed XLHED symptoms. If a girl showed mild or no symptoms, it would be difficult to determine if that was due to the drug or if they would have had those same symptoms without the drug. Conversely, as the boys are consistently more severely affected, the potential risks associated with administering the drug are also more justifiable in boys.
So demonstration of “benefit” linked to the drug is more likely to be achievable in boys. And demonstration that the “benefits” outweighs the risks is also more likely to be achieved in boys.
The Edelife clinical study is therefore only for boys because of the consistent and more severe symptoms experienced in males with XLHED. This consistent starting point will allow any recovery of ectodermal structures and improvement of XLHED symptoms to be attributed to the drug, ER004, which is a synthetic version of the EDA1 protein. This is the most rapid and most likely path to obtaining an approved drug for XLHED.
Once ER004 is approved for use in boys, we will consider administering ER004 to girls.
For more information, visit the EDELIFE website.
Want to stay up-to-date on news and information about the EDELIFE clinical trial? Learn more and sign up for updates.
That’s fantastic news, I’m a 64 year old male and have xlhed ,as so does my brother,we suffer daily and to hear of a breakthrough,so that others can be helped and not suffer the same as us, with the abuse and the not knowing is fantastic,we applaud you 👌
My son who is 28 has Ed and his son who’s 8 has Ed but his daughter who is 5 doesn’t have Ed wondered if they could be part of trails
Hi, Alison! Thanks for reaching out. The potential treatment being studied in the EDELIFE clinical trial is only effective when given in utero, before birth, so unfortunately, your son and grandson would not be able to participate. However, if they were considering having another child, this might be an option for the family. – Veronica
Hi
I have my wife pregnant she is 6 weeks is anything can prevent her from this xlhe?
Please contact our office at info@nfed.org. Thank you.