At his two-day-old checkup, baby Matthew’s pediatrician looked him right in the eyes and said,
“Matthew, you’re changing the future!”
His mother, Beth, reacted, “Oh my gosh! You’re right! He’s only two days old and he’s already changing the future! It’s exciting!”
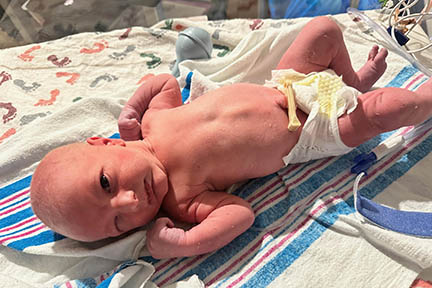
Beth and her husband, Peter, participated in the Edelife Clinical Trial. Their son, Matthew, received three in-utero treatments for x-linked hypohidrotic ectodermal dysplasia (XLHED) in the first study of its kind. The trial aims to confirm results of six boys who were treated and are now sweating normally as well as have other improved symptoms.
We want a normal childhood for Matthew, and when we look at the life that we want him to live – we are in Texas – it’s very hard. We want him to be able to play and participate in sports, go outside without any restrictions or worries. And so if there was a trial that could help us provide that childhood for him, we were going to do it.
A Family Legacy of XLHED

With a family history of XLHED that goes back multiple generations, Beth’s dad, also named Peter, is affected and struggles in the heat. Beth has known since she’s a teenager that she’s a carrier. Her dad would attend NFED Family Conferences and keep Beth informed about the treatment that was being researched and developed. As she got older, Beth followed the NFED Facebook page and stayed up-to-date on the progress.
Preparing for Parenthood
Once the Texas couple got married and decided to start a family, Beth started pursuing the Edelife Clinical Trial. Her first step was to have genetic testing, which confirmed that she did have the EDA gene which causes XLHED. Peter reached out to his father-in-law to better understand how XLHED impacted his life and read lots of information on the Edelife website.
“It came down to that if I was wanting to do it, he was going to support me, and so we went for it,” Beth said. “But, it was hard for him initially because he really did not anticipate going through this. But once it became reality, he got on board pretty fast.”

Once Beth became pregnant, she reached out to NFED Executive Director Mary Fete, who guided her through the process. She connected Beth with Dr. Kathy Grange at the Edelife site in St. Louis. Her local doctor did blood work at 10 weeks of pregnancy which showed the child was a boy. The next step was having an amniocentesis diagnosis, which confirmed their unborn child did inherit the XLHED gene.
The Trial Process Was Easy and Efficient
Beth admitted they were a little nervous about participating in Edelife knowing it was a drug trial.
“Knowing I was one of only a handful of women receiving the drug at that point, there were a lot of unknowns. But, it was also at that point we had learned that there were other women who had baby boys who were sweating and doing well, so you kind of reassure yourself. But yeah, I was nervous because it was new.”
Beth said the logistics of the trial and traveling were not difficult. Ultimately, she made four trips to St. Louis and didn’t have issues getting off work as a pediatric nurse. Since grandpa Peter is affected by XLHED, he participated in Edelife as a control participant.
“My parents actually met me in St. Louis…so they could see the process,” Beth said. “It was really exciting for my dad in particular to be able to be a participant.”

Now, Mathew is eight months old and doing great. Because the trial is still enrolling patients, Edelife researchers have not published any results from the 10 babies like Matthew who have participated so far.
Remembering Her Grandmother’s Strength


In this first year of his life, Beth has thought about her dad’s mom quite a bit and what it must have been like for her as a mom in the 1950s raising a son with a condition for which little was known.
“I can just envision her sitting down with my dad as a baby who had respiratory illnesses and wasn’t tolerating heat well and just struggled as an infant. And I think about her and how she had to push through, and I think she would be so incredibly proud of the work that’s been put into this trial and that if she had had the opportunity to do that for my dad, she would’ve absolutely done it. Anytime I think about Matthew’s future or any of the questions – we still don’t know exactly how HED will affect him – I think about my grandma and just how she handled it. She was a really amazing, positive woman and got through it. If she did it, I can do it!”
Encouragement for Other Families

The process of becoming a mother and participating in Edelife has taught Beth she’s so much stronger than she thought she was. If you are a parent considering the trial, Beth advises to not be scared, ask questions and explore all your options. She encourages you to build your own little ectodermal dysplasia community and reach out to her if you have any questions.
“When you hear about all the possible effects of XLHED, you start to get nervous and you get really worried about what your child’s future is going to look like. How are you going to manage? How is he going to cope? And then you start to realize that it’s maybe not as scary as you think it is, or that it’s not as bad as you imagined.
And the future starts to look really beautiful and exciting. So don’t be afraid. Get excited!…I would do it again in a heartbeat. It’s such an incredible opportunity to completely change the future for your child. Yeah, it’s worth it.”
A Future Without Limits
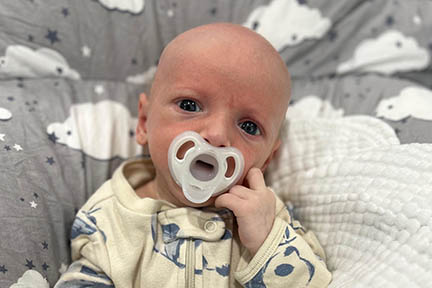
Beth wishes that Matthew grows up to be happy and healthy. Her dream for him is “honestly, that he never feels limited and that he knows that as his mom, as his dad, we did everything we could to provide for him.”
* There were six boys who received the XLHED treatment in a Trial to Cure, a study conducted before the Edelife Clinical Trial. Published findings show they are sweating and have other improved symptoms.
Are You Interested in Edelife?
The Edelife Clinical Trial is enrolling 5-10 more patients at sites in six countries. Find out if you are eligible to participate.
Explore Edelife